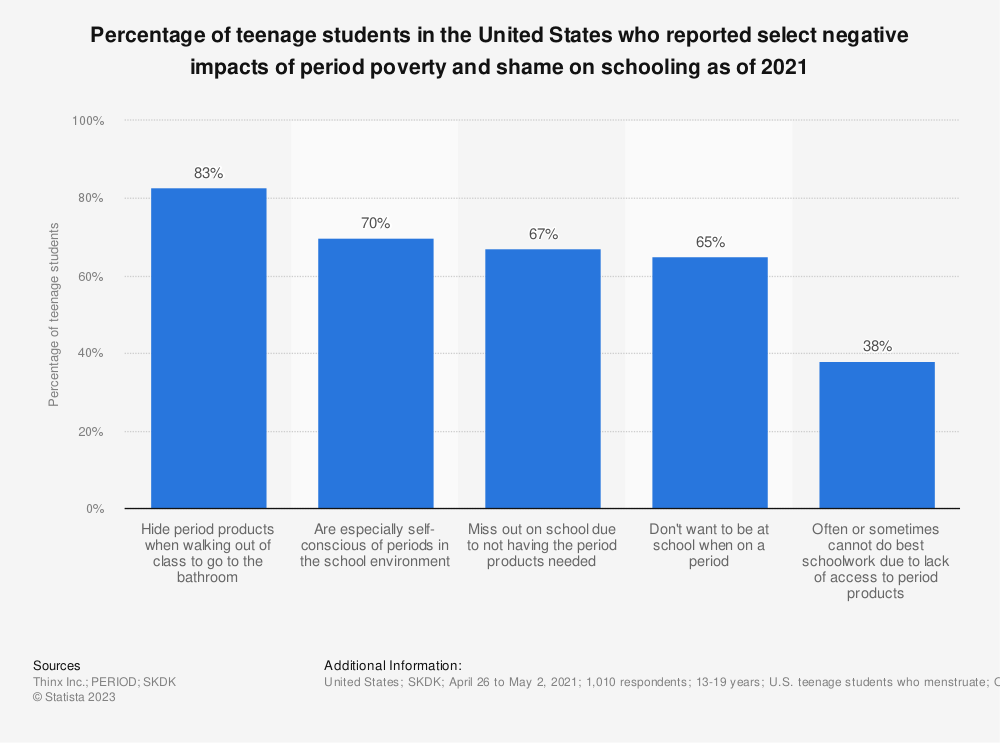
A team of Stony Brook researchers have developed a new understanding of cognitive adaptability and flexibility.
Grigori Enikolopov — a professor in the Department of Anesthesiology in the Renaissance School of Medicine and the Center for Developmental Genetics — and his team researched the impacts of cognitive flexibility and adaptability in both middle- and old-aged mice.
The team aimed to understand the role of adult-born neurons in the mices’ brains in two different regions. The first is the hippocampus, a region involved with memory and learning. The second is the subgranular zone, which carries out neurogenesis, a process when neurons are formed in the brain, in the hippocampus in a situation where the animals would have to learn and adapt to their surroundings.
“There are two studies that we published back-to-back and both of them are aiming at understanding what role adult-born neurons may play in these situations, where cognitive flexibility may be important to adapt to new situations,” Evgeny Amelchenko, a research scientist at Stony Brook University, said.
The work of Enikolopov and his team has been published in the Journal of Neuroscience and Frontiers in Neuroscience. He was the first author for both papers.
The team researched what would happen when neurogenesis decreases. They allowed the mice to age as they normally would, and then compared the results of the old-aged mice to the middle-aged adult mice, who were younger.
The old- and middle-aged adult mice were subjected to the same experiment known as the Morris water maze task, named after Richard G. Morris who first introduced this trial. In this experiment, the mice were placed into a pool and left to navigate various platforms.
Amelchenko said when the pool is placed in a different room, it is surrounded by different spatial and distal cues, which is similar to how humans navigate a location with new surroundings.
“When we’re in a new city, let’s say, we go from the train station to the museum and we try to remember where we need to take left and right turns and so on,” Amelchenko said. “Animals also remember [their] surroundings if we provide them this opportunity, keeping the platform in the same place every time in the pool.”
Enikolopov and his team found that animals with less neurogenesis, whether it was due to radiation or aging, were able to remember the platform location.
When they tested the mices’ ability to locate the platform in the same pool, they discovered that the irradiated mice were able to navigate the new platform location when it was moved. However, the old-aged mice experienced difficulty in finding the new platform location, revealing a deficit in navigation strategies.
Since the irradiated mice responded and adapted well to the moving platforms in the pool, the researchers decided to add another pool that was made of different materials and put it in a new room where it was surrounded by different spatial cues. To increase the complexity of the exercise, they added a local cue by dangling two toys above the pool. One toy signaled the platform location, and if the mice chewed on the toy and looked around, they would be able to find the platform location using local cues rather than distal cues.
They also introduced context discrimination in the Morris water maze task, where the mice had to differentiate between the two pools set up in two different rooms while also having to discriminate the local cues. They found that irradiated mice were normal in spatial and reversal tasks, but deficient in context discrimination tasks. Meanwhile, old-aged mice were normal in spatial tasks, but deficient in reversal and context discrimination tasks.
The team wanted to determine how neurogenesis would be affected after radiation. A protein called “C force” was used to find out how adult-born neurons become activated. The findings show that the 12-week-old cells did not respond well to radiation.
“It tells us that maybe that window when adult-born cells are really responsive to situations of discrimination, some novelty is longer than people believe, until recently,” Amelchenko said. “So, for a longer period of time, they are responsive to situations requiring some adaptation, adjustment of previous knowledge and cognitive flexibility potential.”
When the team labeled adult-born cells in aged animals, they looked for a possible correlation between the number of adult-born cells and the mices’ task performances. They discovered that there is a correlation between the number of the cells and task performance; the more cells that are present, the better the mice perform. This exercise helped the researchers visualize what happens in the brain and how it adapts to these changes.
In both old-aged mice and irradiated mice, the team discovered there is a deficit in animals with decreased neurogenesis, and that cognitive flexibility is temporal, meaning the mice would be able to catch up to the control group with more time and additional training. If the animals are provided with additional training, they would be able to catch up to and perform as well as the controlled groups.
Amelchenko says the team is interested in how and whether these adult-born cells are different, both in terms of input and output connections that they have compared to developmentally generated cells that are present within the dentate gyrus — a region of the hippocampus — and were not generated during adulthood.
The team would also like to see if they could artificially manipulate those cells to suppress activity, as it could result in temporal problems with cognitive flexibility. They speculate if the mice would be able to restore their normal performances in these tasks once their activity is no longer blocked. They could do this by targeting the cells with specific viruses, which would deliver certain proteins that would later allow the previously mentioned manipulations and suppress — not kill — the cells. They would then test their behavior and performance with the cells that are suppressed, then test them again once the cells are unblocked.
“Although we’re aiming at understanding very basic processes for mammals, the limitation of this study in mice, in laboratory animals, is that these are animals that spent their whole life in an artificial environment, in cages, in animal facilities,” Amelchenko said. “And the potential role for neurogenesis in these mice may be very different from animals, or rodents, caught in the wild.”
Different mammal species share similar behaviors and interactions with their environment. However, each species evolves differently and has unique ways of interacting with their environment.
The animals’ environment shapes their evolution as it forces species and their brains to adapt to new environments. An adaptation in the brain results in a change in behavior.
In the case of adult-born neurons, these cells have unique roles depending on their environment and what species they occupy. Therefore, adult-born neurons in wild animals and caged animals would be different due to their different environments.